Basics for measuring the gas concentration in air
Why is the Measurement of Room Air Quality So Important?
An unsatisfactory room air quality of indoor rooms (e.g. in offices) can easily cause tiredness, poor powers of concentration and even diseases to people. Indicator for the room air quality is the concentration of specific gases in air. The most important ones include:
- Carbon dioxide (CO2)
- Carbon monoxide (CO)
- Oxygen (O2)
- Ozone (O3)
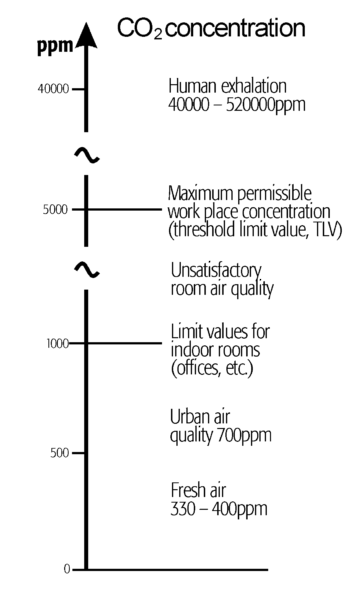
CO2-Concentration
An important criterion for the evaluation of the room air quality is the CO2 concentration. A CO2 concentration, which is too high due to insufficient ventilation, is experienced as stale or stagnant air. The illustration above shows the range of CO2 concentrations that are relevant to a human.
CO-Concentration
CO is produced when carbon is only partially combusted (fuel). CO is very dangerous for humans because it is at the same time highly toxic - but invisible and odorless. Reasons for the production of CO in various combustion processes:
- deficiency of air
- too high excess of air
- too early cooling down of flame
Effects of CO in the ambient air on the human body
| CO concentration | Inhalation period and consequences | |
|---|---|---|
| 30 ppm | 0.003% | Maximum concentration in the workplace per 8-hour shift (German MAK value) |
| 200 ppm | 0.02% | Slight headache within 2 to 3 hours |
| 400 ppm | 0.04% | Headache within 1 to 2 hours, first in the forehead and temples, then spreading to the whole head |
| 800 ppm | 0.08% | Dizziness, nausea, and twitching limbs within 45 minutes, unconsciousness within 2 hours |
| 1600 ppm | 0.16% | Headache, dizziness, nausea within 20 minutes, death within 2 hours |
| 3200 ppm | 0.32% | Headache, dizziness, nausea within 5 to 10 minutes, death within 30 minutes |
| 6400 ppm | 0.64% | Headache and dizziness within 1 to 2 minutes, death within 10 to 15 minutes |
| 12800 ppm | 1.28% | Death within 1 to 3 minutes |
Applications
- measurement, control, and warning system in garages,
- monitoring of room air quality with respect to maximum permissible workplace concentration (MAK value)
- monitoring of outside air or of protected air systems in domestic and large public shelters.
O2-Concentration
The inhaled air consists of vital oxygen at a ratio of 1:5. Oxygen is required for all oxidation processes; for combustion processes, as well as for silent oxidations. Examples include the rusting of iron, oxidations, which occur in living processes, or the decomposition of organic material. Additionally, all combustion processes that release energy require this gas, for example, heating systems or aircraft engines. However, oxygen is also bound with any type of noxious fires such as forest and heath fires. Due to the permanent cycle of assimilation and photosynthesis in green plants when they are subject to sunshine, oxygen is continuously re-formed from carbon dioxide. The balance between oxygen consumption and oxygen production is disturbed by the continuously increasing combustion of fossil combustibles.
Therefore, many areas require control measurements of the oxygen content in the air, e.g. in air condition systems, air purifiers, oxygen rectifiers, greenhouses and oxygen incubators, as well as for exhaust emission tests, e.g. in the automotive industry.
O3-Concentration
he ozone contained in the earth’s atmosphere forms at altitudes of approximately 30km. It provides a protective shield around the earth and filters out approximately 50% of the solar UV radiation, particularly the short-wave range, which is dangerous for living organisms. However, ozone is toxic and an extremely aggressive trace gas that can cause major burns in human mucous membranes when breathed in high concentrations. Therefore, control measurements for the ozone content in air must be performed in many areas, e.g. leakage tests in industry, protection of health and safety standards at work, mobile-based air quality measurements or for providing environmental data on advertising displays etc.
Calculation Formulae
The following formulae are used for converting the O3 measured value from ppb to µg/m³, depending on the current atm. pressure and the temperature.
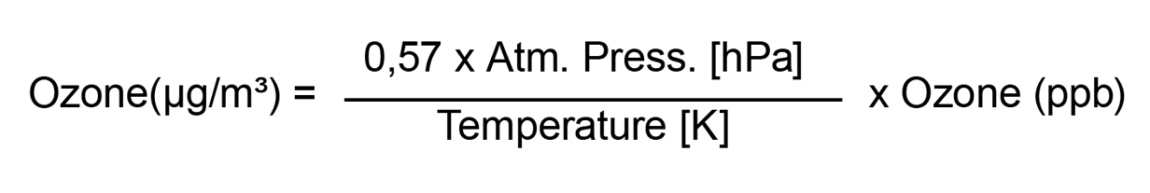
Example:
20°C and 1013 hPa = factor 2
Ozone (µg/m³) = 2 x Ozone (ppb)
This is the nominal value for conversion from ppb to µg/m³.